Calcium carbonate, as an important inorganic filler, is widely used in polymer composite materials, coatings, papermaking, and other fields. However, its strong surface hydrophilicity and poor interfacial compatibility with organic matrices limit performance optimization. Traditional chemical modification methods often rely on coupling agents or surfactants. They have limitations such as complex processes and high environmental loads. However, the surface irradiation modification of calcium carbonate can solve this problem.
Irradiation modification technology involves irradiating calcium carbonate particles with high-energy rays (such as gamma rays or electron beams) or plasma to induce controllable reconstruction of their physical and chemical surface structures. Irradiation energy can create lattice defects in calcium carbonate, generate active free radicals, promote the rearrangement of functional groups such as surface hydroxyl groups, and form micro-nano rough structures through etching. These modifications significantly improve the interfacial bonding strength with the polymer matrix.
Surface Irradiation Treatment of Calcium Carbonate: Graft Polymerization of Acrylamide on Calcium Carbonate Powder
1. CaCO₃ Irradiation Modification
1.1 Pretreatment and Pre-irradiation of CaCO₃ Powder
The powder was dried at 120–140°C for 2 hours to remove moisture and other volatile components. Then, the pretreated powder was weighed quantitatively and pre-irradiated with a high-energy electron beam under nitrogen protection. The irradiated powder was very stable. Its ability to initiate monomer grafting was hardly affected by storage at room temperature for three days.
1.2 Grafting Polymerization of Acrylamide on Pre-irradiated Powder
Accurately weigh the pre-irradiated powder, add a certain amount of acrylamide pre-dispersed with water, and carry out graft copolymerization. After the reaction, the grafted copolymer was extracted with acetone for 8 hours, vacuum-dried to constant weight, and set aside.
2. Results and Discussion
2.1 CaCO₃ Irradiation Modification
2.1.1 Effect of Pre-irradiation Dose on the Formation of Organic Structure on the Surface of Filler
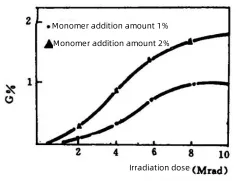
Figure 1: Effect of irradiation dose on the formation of organic structure on the surface of CaCO₃
The effect of irradiation dose on the content of organic structure formation on the surface (G%) is shown in Figure 1. As can be seen from Figure 1, when the irradiation dose is less than 6 Mrad, the content of organic structure on the surface (G%) increases significantly with the increase of irradiation dose. When the dose exceeds 6 Mrad, the change becomes less pronounced, and it reaches equilibrium after 8 Mrad.
2.1.2 Effect of Active Monomer Dosage on Surface Organic Content
When the irradiation dose is kept constant, varying the amount of monomer added can also affect the organic content formed on the surface. The relationship is shown in Table 1. As can be seen, as the monomer dosage increases, the surface organic content (G%) also increases. However, the monomer utilization efficiency (grafted AAM weight / total AAM weight) decreases.
This indicates that not all added active monomers participate in the surface reaction and form a coating layer on CaCO₃. This may be attributed to the decreasing relative concentration of monomers. Because as the grafting reaction progresses, and the formation of the grafted layer may hinder the further diffusion of monomers into it.
Table 1: Effect of Monomer Dosage on Surface Organic Formation Content (Irradiation Dose: 8 Mrad)
| No. | A0 | A1 | A2 | A3 | A4 | A5 |
| Monomer addition amount % | 0 | 1 | 2 | 3 | 5 | 10 |
| Organic formation content G% | 0 | 1 | 1.7 | 2.5 | 3.4 | 6.7 |
2.2 Structure and Surface Properties of Modified Calcium Carbonate
2.2.1 Infrared Spectrum of Modified Calcium Carbonate
Figure 2 shows the infrared spectra of unmodified calcium carbonate, modified calcium carbonate, acrylamide, and polyacrylamide.
By comparing the spectra in Figure 2, the following observations can be made:
(1) Polyacrylamide and modified calcium carbonate exhibit no absorption around 1600 cm⁻¹. It corresponds to the double bond peak of acrylamide.
(2) A characteristic amide absorption peak appears at 1658 cm⁻¹ in the modified calcium carbonate, which is also present in the polyacrylamide spectrum.
Additionally, the absorption peak near 1425 cm⁻¹ shifts to approximately 1443 cm⁻¹.
These phenomena confirm that acrylamide has reacted with the CaCO₃ surface, indicating a clear chemical interaction between acrylamide and calcium carbonate.
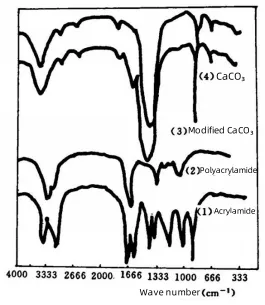
Figure 2 Infrared spectrum of irradiated modified calcium carbonate
2.2.2 Surface Properties of Modified Calcium Carbonate
Figure 3 illustrates the contact angle and oil absorption rate of irradiated and grafted modified CaCO₃ in liquid paraffin.
It can be seen that as the surface organic content (G%) of CaCO₃ increases, the oil absorption rate rises significantly while the contact angle decreases. This indicates a substantial improvement in lipophilicity, which enhances the dispersion of filler particles in the polymer matrix. It also strengthens the interaction between filler particles and polymer molecules, thereby improving the overall performance of the filled composite system.
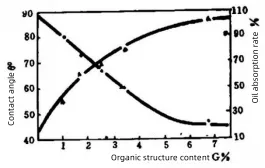
Figure 3: Contact angle and oil absorption rate of irradiated modified calcium carbonate
2.2.3 Bulk Density and Particle Size of Modified Calcium Carbonate
Table 2 shows the relationship between the average particle size and bulk density of modified calcium carbonate and its surface organic content.
As the organic content on the CaCO₃ surface increases, the bulk density decreases, while the average particle size shows a slight increase. The presence of more organic components reduces the surface polarity of CaCO₃, weakening the interparticle interactions and aggregation, making the particles more loosely packed.
At the same time, the reduced polarity lowers the interfacial tension between CaCO₃ and liquid paraffin, resulting in a smaller contact angle.
Table 2: Particle Size and Bulk Density of Modified CaCO₃
| No. | A0 | A1 | A2 | A3 | A4 | A5 |
| Organic structure content | 0 | 1 | 1.7 | 2.5 | 3.4 | 6.7 |
| Average particle size, µ | 4.7 | 5.0 | 5.1 | 5.2 | 5.2 | 5.8 |
| Stacking density g/cm³ | 0.33 | 0.30 | 0.30 | 0.28 | 0.28 | 0.27 |
3. Conclusion
For the surface irradiation modification of calcium carbonate, there are below conclusions.
1. Electron beam pre-irradiation can generate an organic coating on the surface of calcium carbonate, transforming its properties from hydrophilic to lipophilic. This significantly reduces the interfacial tension with liquid paraffin and lowers the contact angle. As a result, the compatibility of irradiation-modified CaCO₃ with polymer materials is greatly enhanced.
2. The formation of organic matter on the surface of CaCO₃ is influenced by several factors, including irradiation dose, monomer dosage during pretreatment, and irradiation time.
3. The irradiation grafting reaction primarily follows a free radical reaction mechanism.
About Epic Powder Machinery
Epic Powder Machinery is a leading manufacturer of ultrafine powder processing equipment, with decades of experience in precision grinding, classification, and surface modification technologies. We offer customized solutions for calcium carbonate irradiation modification and other advanced material applications. With in-house R&D and European-standard quality, Epic Powder is your reliable partner for improving material performance and production efficiency.
Contact us today to learn more about how our solutions can enhance your powder processing needs.

