1. Why Does Dust in the Air Always Cluster?
When the sun shines through the window, why does the dust floating in the air always seem to fly in clumps? Once flour gets wet, it tends to clump together, making it difficult to disperse, even if stirred. In hazy weather, why does the air pollution seem to thicken overnight? Behind these phenomena lies a characteristic of particles: they tend to cluster together, much like people gather together for warmth in the cold wind. This process is known as particle agglomeration.


2. Why Do Particles Agglomerate?
There are three main reasons why particles agglomerate:
2.1 Van der Waals Force:
Van der Waals force can be thought of as a kind of silent attraction. Even when particles are not charged, they form brief, small magnetic poles due to the instantaneous movement of electrons inside the molecules, which attract them to each other.
In fact, van der Waals force is an intermolecular force.
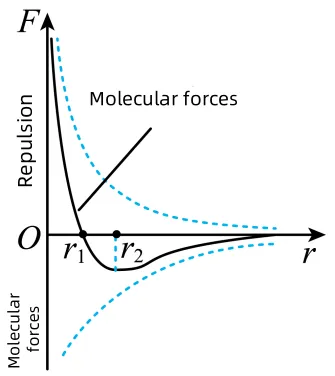
Just like when two pieces of paper come close together and are attracted by static electricity, even without an obvious charge or magnetism. Similarly, when you approach a TV screen, your hair flies up due to the weak molecular force at work.

For particles with a diameter of less than 10 microns, van der Waals force often outweighs gravity, which is why fine particles such as PM2.5 and PM1.0 tend to stick together and are difficult to remove.

2.2 Liquid Bridge Force:
When there is moisture in the air, the surface of the particles easily absorbs water vapor, forming a liquid bridge, much like a drop of water connecting two small balls.
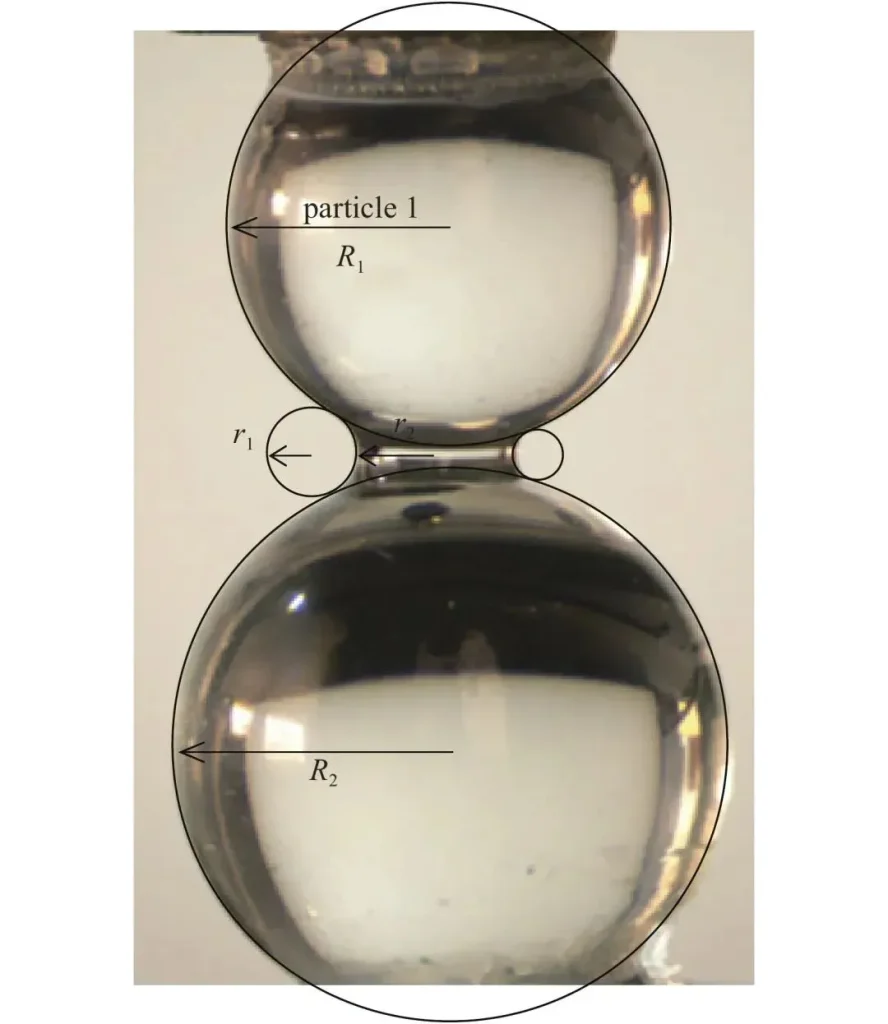
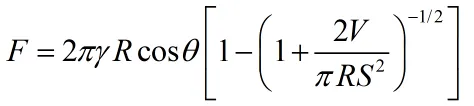
For example: two dry grains of sand will not stick together, but if they are slightly wet, they can clump together by water. Dry sand cannot build a castle, but wet sand can build a hill—this is the principle in action.

Typical scenarios: Kitchen seasonings clump due to moisture, washing powder clumps due to moisture absorption, and reduced dust on rainy roads.
2.3 Collision and Agglomeration:
As particles move through air or water, many relative movements and collisions occur. If conditions are right (e.g., if the speed isn’t too fast, and the surface has adsorption or a liquid film), the particles may stick together after collision, forming larger particles.
Imagine a group of people sitting in a shaking bus. If there are too many people and the bus shakes too much, they often collide with each other and hold onto one another to stand firm. This unintentional adhesion is similar to the collision and agglomeration of particles.
The speed of this type of agglomeration depends on particle concentration, flow field speed, and turbulence intensity. The more violent the flow and the greater the number of particles, the higher the probability of collision.
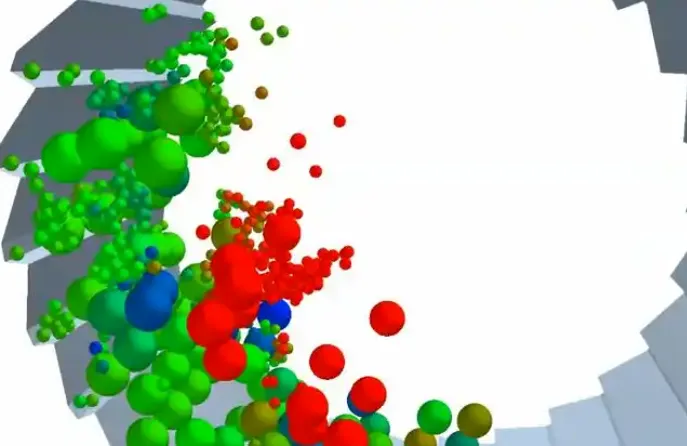
3. How to Study Particle Agglomeration in Simulation?
In engineering, we often use Computational Fluid Dynamics (CFD) and Population Balance Models (PBM) to simulate the agglomeration process of particles.
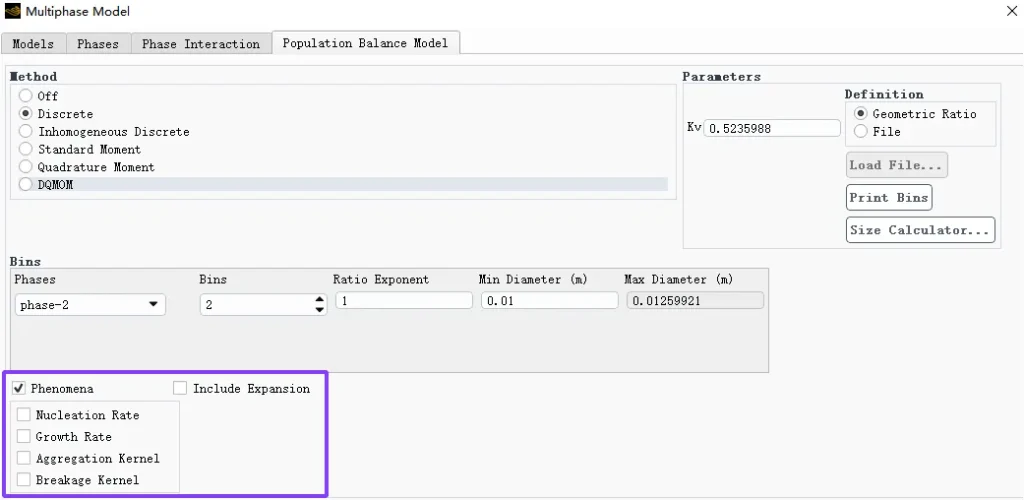
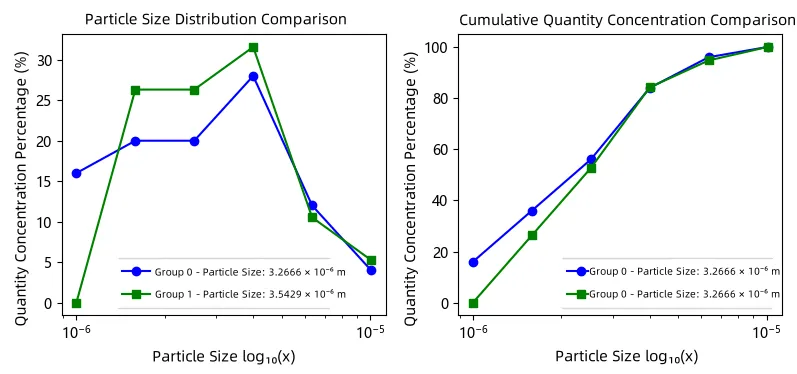
PBM does not simulate the trajectory of each particle, but instead describes the evolution of a “particle ensemble” in the particle size space:
How the number of particles decreases over time (because more particles merge into larger ones)?
How the average particle size increases?
How the particle size distribution (PSD) changes shape: from a sharp single peak
→ gradually widening or forming double peaks?
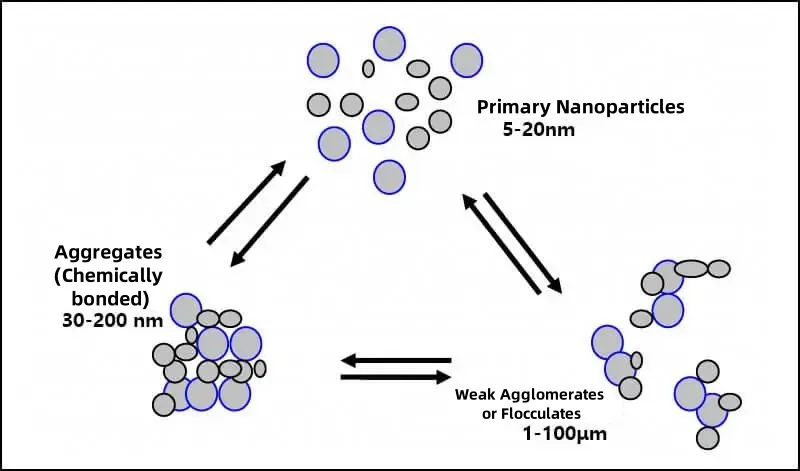
4. Which Industries Are Affected by Particle Agglomeration?
4.1 Agglomeration Is common in Nature
Formation of Sand Dunes: Sand particles adhere to each other and accumulate during wind transport. Soil Aggregate Structure: Clay and organic matter combine to retain water and fertilizer. Cell Aggregation: Intermolecular forces in organisms promote tissue formation.

4.2 Industrial Applications
Agglomeration can be both a blessing and a curse in industrial applications.
| Scenario | Impact | Is agglomeration required? |
| Air Purification | Particle size increases → Easier to filter | ✔ Yes |
| Haze Control | Agglomeration leads to easier settling → Air quality improvement | ✔ Yes |
| Pharmaceutical Formulations | Uneven particle size → Unstable dosage | ✕ No |
| Spray Drying | Reunion causes nozzle clogging and clumping | ✕ No |
| Chemical Reactor | Reduced reaction surface & poor heat transfer | ✕ No |
4.3 Anti-Agglomeration Technology
After understanding the mechanisms behind agglomeration, engineers have developed various solutions to prevent it.
| Anti-Caking Technology | Mechanism | Application Scenarios |
| Surface Coating | Forms an isolation layer with silica | Milk powder, instant coffee |
| Fluidized Bed Drying | Hot air creates particle suspension | Pharmaceutical granulation |
| Antistatic Agents | Reduces surface resistance | Plastic particle processing |
| Ultrasonic Vibration | Destroys liquid bridge structures | Powder screening equipment |

Therefore, whether the agglomeration behavior of particles can be accurately simulated and predicted is a key technology for industrial process control.
Epic Powder
Epic Powder Machinery specializes in advanced powder processing technologies, offering cutting-edge solutions for particle size reduction and agglomeration control. With years of expertise and a commitment to innovation, we provide tailored equipment for various industries, ensuring optimal performance and efficiency in particle processing. Trust Epic Powder to help you optimize your production processes and achieve superior results.

