The name Kaolin originates from Gaoling Village, near Jingdezhen in Jiangxi Province, China. During the Ming and Qing dynasties, Jingdezhen was the renowned porcelain capital of China. The essential white clay used for porcelain production was primarily mined from the Gaoling area, hence the name “Gaoling Tu”, meaning “earth from Gaoling,” was given to this mineral. Later, Western missionaries and scholars transliterated this name into Kaolin. At Epic Powder, our HTS315 Air Classifier is engineered to deliver unmatched precision in kaolin classification. The precision of kaolin classification is paramount for achieving desired cosmetic properties. Modern kaolin classification techniques, particularly advanced air classification, have superseded traditional methods in efficiency and accuracy, enabling the production of tightly controlled particle size distributions crucial for high-performance applications.

I. Properties of Kaolin
1. Kaolin Structure
Kaolin is a representative clay mineral of the kaolinite group, possessing a 1:1 layered crystal structure. Each layer consists of one silica tetrahedral sheet (T) bonded to one alumina octahedral sheet (O), referred to as a T-O layer.
Individual crystal layers are tightly bonded via the silica tetrahedral and alumina octahedral sheets. These layers are connected by hydrogen bonds, preventing easy insertion of water molecules between them. Consequently, kaolin is non-swelling and has a low cation exchange capacity (CEC).
Kaolin crystals are typically fine, platy/hexagonal platelets, exhibiting distinct anisotropy. Regarding surface characteristics: the silica tetrahedral face carries oxygen anions, usually negatively charged above pH 4. The alumina octahedral face exposes hydroxyl groups, positively charged below pH 6 and transitioning to negative above pH 8.
Kaolin vs. Montmorillonite
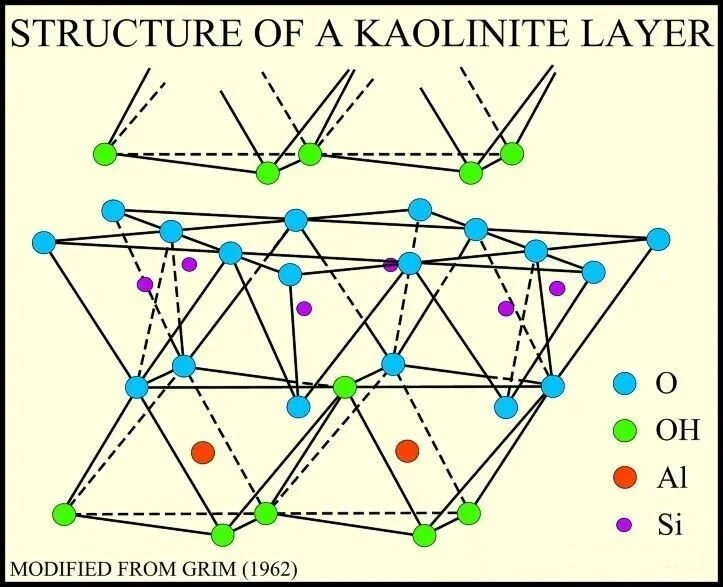
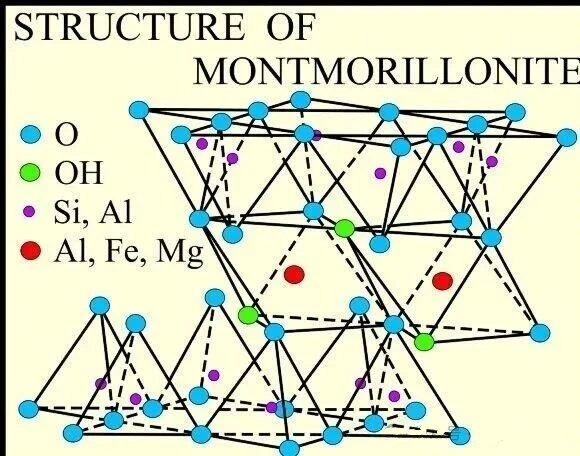
| Mineral Type | Structure Characteristics | Properties | Application Scenarios |
| Kaolin (1:1, T-O) | Layered structure, non-swelling | Low CEC, mild thixotropy | Mud masks, loose powders, matte lipsticks |
| Montmorillonite (2:1, T-O-T) | Layers can admit water and ions, capable of swelling | High CEC, strong swelling, significant thickening | Powerful thickeners, colloidal anti-settling applications |
SEM Image of Kaolin
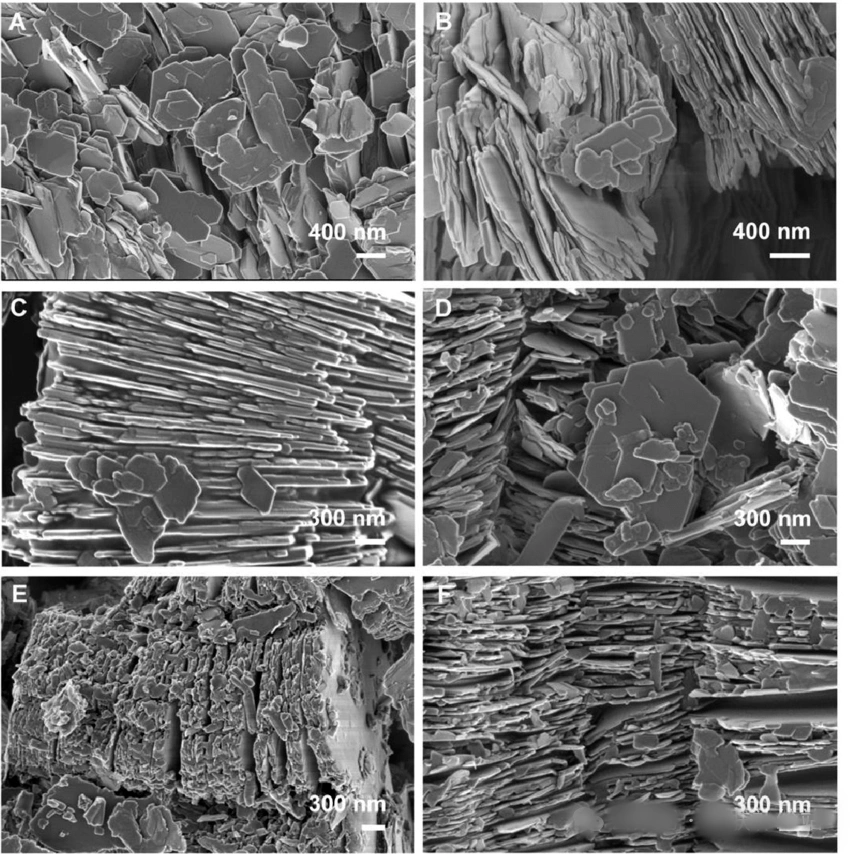
SEM image of kaolin particles displaying the typical layered plate-like morphology. This platy structure imparts excellent coverage and adsorption properties to kaolin, while also conferring thixotropic behavior to its aqueous suspensions. Note: The plate-like structures in the image are not single T-O layers but stacks of many parallel T-O layers.
The diverse functionality of kaolin in cosmetics – from oil adsorption to texture enhancement – is fundamentally governed by its particle size distribution, which is precisely defined through rigorous kaolin classification processes. This makes effective kaolin classification the critical first step in tailoring kaolin for specific cosmetic formulations.
2. Physicochemical Properties
Hydrophilicity/Hydrophobicity
Natural kaolin has a strongly hydrophilic surface, easily wetted by water. This affinity for water stems from exposed hydroxyl groups on the crystal surfaces and negative charges within the layers. In contrast, hydrophobic powders like talc tend to float and resist wetting in water.
Kaolin can be modified via surface treatment. For instance, silylation (e.g., with triethoxycaprylylsilane) can impart hydrophobicity, making it suitable for dispersion in oil-based systems. Calcined kaolin, having reduced surface hydroxyl groups, exhibits slightly increased hydrophobicity but generally remains hydrophilic overall.
Oil Absorption Value and Water Absorption
Kaolin possesses excellent oil absorption capacity, which is highly valued in color cosmetics for oil control. The oil absorption value is commonly measured by the ASTM D281 method, expressed as the amount of oil absorbed per 100g of powder. Fine-grade kaolin typically has an oil absorption range of 45–60 g/100g. Calcined kaolin, which develops a porous structure, can exhibit even higher oil absorption values.
Regarding water absorption, kaolin can absorb approximately 0.3–0.5 times its own weight in water, forming a plastic paste. This property makes it easy to apply in mud masks without dripping. In comparison, bentonite has a much higher water absorption capacity (capable of swelling several times its volume).
It’s important to note that the adsorption performance of kaolin is significantly influenced by particle size and specific surface area – finer powders generally have higher oil demand. Therefore, selection should balance oil absorption requirements with skin feel (excessively high absorption can lead to a dry, dragging sensation).
Whiteness and Coverage
Kaolin is inherently a white powder, but its whiteness depends on impurity content (e.g., iron oxides) and processing purity. Cosmetic-grade kaolin is usually bleached and purified, achieving a whiteness (ISO brightness) of 85–95%. For example, one Indian kaolin reports an L-value of 95±0.5 and a whiteness index of 87±1.
Kaolin has a relatively moderate refractive index. The principal refractive indices for the monoclinic crystal are approximately 1.56 (nα ≈ 1.553–1.565, nγ ≈ 1.569–1.570), close to that of the skin stratum corneum (~1.55). Consequently, kaolin powder applied to the skin exhibits a degree of transparency. It’s lacking the strong coverage provided by high-refractive-index pigments like TiO₂ (n ≈ 2.7).
However, the platy morphology of kaolin particles provides light shielding and scattering. Incorporating a certain amount of kaolin into foundations or compacts can enhance coverage/matte effects without the pronounced whitening caused by titanium dioxide.
Furthermore, kaolin can also act as a jmatteifying agent in formulations, reducing product shine. When evaluating coverage, the difference in powder reflectance on black and white contrast cards can be measured. Kaolin generally falls between talc (more transparent) and titanium dioxide (strong coverage).
Density and Specific Gravity
The true density of kaolin is approximately 2.6–2.63 g/cm³. In cosmetics, its bulk density often characterizes powder fluffiness. Lightweight kaolin, often achieved through advanced kaolin classification processes like air classification. It has a lower bulk density around 0.3–0.5 g/cm³, whereas unprocessed heavy kaolin can be >0.8 g/cm³. In loose powder formulations, bulk density affects powder dusting and skin feel. Typically, fluffier powders feel lighter, but require higher compression force for pressing into compacts. During experimental formulation, variations in bulk density between different kaolin batches should be noted, adjusting volume ratios if necessary to ensure consistent compaction.
pH Value (Suspension)
Kaolin forms slightly acidic suspensions in water. Typically, uncalcined natural kaolin in a 20% w/v pure water suspension has a pH around 4–6. This acidity arises from H⁺ ions released from the kaolin surface into the water phase.
Due to dehydroxylation, calcined kaolin suspensions often have a slightly higher pH, closer to neutral (6–7).
The pH of the formulation system crucially affects kaolin dispersion and stability: around pH ~4–6, kaolin tends to flocculate (positive edge-negative face attraction), forming a thixotropic gel; under alkaline conditions (pH >9), all kaolin particle surfaces carry negative charges, repel each other, resulting in more stable suspensions but lower viscosity. Therefore, in weakly acidic formulations (e.g., acid-based mud masks), kaolin can easily thicken, and the degree of flocculation should be evaluated beforehand. In alkaline formulations (e.g., soap-based cleansers), kaolin might settle more easily, requiring the addition of suspending agents.
Note: “Flocculation” here refers to the reversible, loose particle network formed by edge-positive ↔ face-negative attraction under specific pH and ionic conditions, causing the system to thicken at rest and shear-thin.
Rheological Behavior
Kaolin water dispersions exhibit shear-thinning and thixotropy. At higher kaolin concentrations (>10–15% w/w), the paste develops a yield stress at rest, requiring application of a certain stress to initiate flow. This stems from the loose network structure formed by edge-face electrostatic interactions between kaolin particles under static conditions.
• Yield Stress
◦ Definition: The minimum stress required to initiate flow in a fluid or paste.
◦ Intuitive Understanding: Like toothpaste standing on a brush without slumping – this is due to yield stress; it flows only when squeezed.
◦ Significance: Higher yield stress allows the material to maintain its shape at rest, resisting settling or flowing.
• Thixotropy
◦ Definition: The property where a material’s viscosity decreases under shear (stirring, application) and gradually recovers upon standing.
◦ Intuitive Understanding: Like honey or mud masks – they feel thinner when stirred but thicken again after standing.
◦ Significance: Thixotropy makes cosmetics easy to apply but allows them to stay in place after application without immediate running.
• Experimental data show that yield stress increases approximately exponentially with solid content; e.g., a 30% kaolin paste has a significantly higher yield stress than a 20% paste.
• Furthermore, pH and ionic strength significantly affect rheology: yield stress and thixotropic loop area peak at low pH (e.g., around 4). Flocculation weakens and yield stress decreases as pH rises above 8.
• Simultaneously, electrolytes (especially polyvalent cations like Ca²⁺, Mg²⁺) compress the electrical double layer, inducing rapid particle aggregation, significantly increasing yield stress and viscosity. Therefore, formulations containing high salt concentrations (e.g., electrolytes in astringents or hydrating toners) require caution regarding their potential to thicken kaolin pastes.
Adding thickeners or colloidal stabilizers (e.g., xanthan gum, cellulose derivatives) can further adjust the yield stress and thixotropic recovery rate of kaolin suspensions to meet different application needs (e.g., masks require non-dripping application but easy spreadability. Liquid foundations may require lower yield stress for easy dispensing).
3. Origin and Classification
Raw kaolin ore typically requires purification and classification to meet cosmetic-grade specifications. Common processes include:
(1) Washing: Utilizing differences in specific gravity between kaolin and quartz/feldspar to remove sandy material via sedimentation in water.
(2) Grinding/Deagglomeration: Using attritors or mills to break down large aggregates into primary crystals.
(3) Centrifugal Classification: Separating products into different particle size grades (e.g., D50 2 µm, 5 µm, 10 µm) based on settling velocity. Modern air classification systems, such as the HTS315 air classifier, are highly effective for achieving precise particle size cuts essential for specific cosmetic applications, directly impacting properties like feel, opacity, and oil absorption. Proper kaolin classification is a critical step in quality control.
(4) Chemical Bleaching: Using reducing agents like hydrazine or sulfur dioxide to remove iron oxide impurities, improving whiteness.
(5) Calcination: Heating kaolin to 500–800°C to remove water of crystallization, producing metakaolin or amorphous calcined kaolin.
(6) Surface Treatment: As mentioned, using silanes or metal soaps to enhance hydrophobicity or improve compatibility with oil phases.
Impact of Processing Differences: Washed kaolin (uncalcined) retains its crystal structure, offering higher plasticity, suitable for mud masks and systems requiring spreadability. Calcined kaolin, due to collapsed lattice structure, forms brittle, porous particles with higher oil absorption but slightly poorer suspension stability (lacking the thixotropic network). The table below summarizes typical physical parameters of kaolins from different origins and treatments.
Table 1: Comparison of Physical Parameters for Kaolins from Different Origins/Processing Methods
| Type / Origin | Key Characteristics | D50 (µm) | Specific Surface Area (m²/g) | Whiteness (ISO %) | Oil Absorption (g/100g) | pH (5% Paste) |
| Jiangxi, China (Washed) | Natural fine particle size, minor iron impurities | ~2–4 | 15–25 | 85–90 | 45–55 | 4.0–5.5 |
| Georgia, USA (Soft) | Small platelet size, high brightness | ~1–3 | 20–26 | 90–93 | 50–60 | 5.0–6.0 |
| Georgia, USA (Calcined) | Porous, high brightness, increased hardness | ~1–2 | 30–50 | 93–95 | 80–90 | 6.0–7.0 |
| Brazil, Amazon | Very low impurities, “Amazonian White Clay” | ~3–5 | 10–20 | >93 | 40–50 | 5.0–6.0 |
| USP Light Kaolin | Ultra-fine grinding, may contain dispersants | ~1 | 20–30 | 85–90 | ~60 | 4.5–7.5 |
| Surface Treated (e.g., Silane) | Silane-modified, hydrophobic, lipophilic | Base mat. dependent | Similar to base material | 85–92 | Similar to base material | – |
Note: Data compiled from various supplier public information and literature. Whiteness is ISO Brightness or converted Hunter L value. For specific grades, refer to supplier datasheets.
Optimizing Kaolin Performance through Precise Kaolin Classification
The selection of the right kaolin grade hinges on understanding its properties, which are profoundly influenced by particle size distribution. Implementing rigorous kaolin classification protocols, potentially utilizing equipment like the HTS315 air classifier, ensures batch-to-batch consistency and allows formulators to target specific performance attributes, whether for oil control, texture, or coverage in the final cosmetic product.
II. Application of Kaolin in Cosmetics
Kaolin finds extensive application in cosmetics, spanning color cosmetics, skincare, and personal care. Its primary functions include adsorbing oil/sweat, improving texture and skin feel, enhancing coverage and matte effects, and stabilizing suspensions.
1. Application in Different Products
Color Cosmetics
In loose powders and setting powders, kaolin is often used at 5–15% as an oil-absorbing filler, providing oil-control, long-lasting wear, and soft-focus matte effects. Its fine particles adsorb excess sebum from the skin’s surface, reducing shine. Because its refractive index is close to that of skin, it avoids a chalky white cast.
Kaolin is also used in pressed powder compacts and foundations, typically added at 10–30%, acting as a bulking filler and coverage aid. Compared to talc, kaolin has higher oil absorption, improving the oil-control feel for oily skin types, but excessive amounts can make the powder too dry and less silky. Therefore, it’s often combined with mica or silica.
Creamy color cosmetics like concealers and contour products sometimes contain 3–10% kaolin, utilizing its oil-absorbing properties to prevent oil migration and increasing paste consistency to prevent settling.
Note: In eye makeup like eyeshadows, kaolin content is generally kept below 5–8% to avoid affecting color payoff and skin adherence, though its oil absorption can help prevent creasing on oily eyelids.
Cleansing Products
Cleansing masks/mud masks are one of the most classic applications for kaolin. In typical formulations, kaolin is blended with other clays (e.g., bentonite, montmorillonite), with contents potentially as high as 20–40% (in aqueous paste form). It acts as the primary adsorbent in mud masks, capable of penetrating pores to adsorb oil, dirt, and impurities, achieving pore cleansing and degreasing.
In exfoliating scrubs, fine kaolin powder can serve as a mild abrasive, helping to remove dead skin cells while adsorbing sebum, without causing micro-scratches like some nut shells.
In daily use facial cleansers, 1–5% kaolin is sometimes added to enhance cleansing power and texture: kaolin can synergize with surfactants to remove oil and grease, and impart a smooth, rich feel to the cream.
Some oil-control lotions/primers contain trace amounts of kaolin (1–3%) for long-lasting sebum adsorption to maintain a matte finish, while also providing some slip for easy application.
Hair and Body Care
Kaolin is increasingly used in dry shampoo sprays/powders. It can be added at 10–30%, mixed with corn starch, silica, etc. As the dry shampoo base powder, effectively adsorbing oil from hair strands and the scalp, leaving hair voluminous and fresh. Kaolin particles are smaller than talc, leaving less white residue and are easier to brush out. This makes it popular in many water-free shampoo products.
In antiperspirant/deodorizing products (e.g., underbody powders, foot/deodorizing powders), kaolin helps reduce body odor by absorbing moisture from sweat and adsorbing odor molecules (e.g., short-chain fatty acids). Typical usage is 15–30%, combined with sodium bicarbonate, zinc oxide, etc., for a dual physical adsorption and antibacterial approach.
It’s worth noting that due to its ability to adsorb ammonia and organic amines, kaolin can help mitigate sweat and foot odor. Compared to odor adsorbents like diatomaceous earth, kaolin is mild and has low skin irritation potential.
Sun Care
Kaolin itself is not a primary UV filter but can act as a physical opacifying aid. Studies show that incorporating fine kaolin into sunscreen formulations can increase UV scattering and absorption. Simultaneously, kaolin imparts a dry skin feel, reducing the greasiness of high SPF formulas.
However, kaolin’s protective effect is far less than that of specialized UV pigments (TiO₂, ZnO), and its inclusion is more for assistive role and texture considerations
Lipstick
In matte lipsticks, lip muds, and other high-oil paste systems, kaolin is often used as a structurant and matting powder. Usage is typically 3–10%. It can absorb some oil within the paste, preventing sweating (oil exudation), and increase paste hardness to prevent deformation. Kaolin also imparts a matte finish, reducing the inherent shine of the lipstick. Because kaolin particles are fine, they can impart a slight gritty feel; thus, it should be used with smooth powders like talc and mica to ensure smooth application.
Creams & Lotions
In skincare formulations like creams and lotions, kaolin is used at low levels (generally <2%), primarily for tactile modification and thickening/suspension assistance. Additionally, its adsorptive properties can be utilized in acne creams to adsorb inflammatory exudates from the skin’s surface, aiding in drying and healing blemishes (some acne patches utilize this principle of clay absorbing fluids).
2. [Key] Formulation and Process Considerations
Dispersion Order and Pre-wetting
Kaolin is a fine powder prone to agglomeration and clumping if added directly to the water phase. Kaolin classification and correct dispersion sequence and method are crucial.
It is generally recommended to pre-wet the kaolin with a small amount of liquid to form a slurry before incorporating it into the main batch. For aqueous systems, glycerin or propylene glycol can be used in a 1:1 to 1:2 ratio with kaolin to pre-mix, ensuring each particle surface is wetted first, avoiding direct contact with water causing clumping.
If pre-dispersion isn’t feasible, kaolin can be slowly sifted into the aqueous phase under moderate to high-speed stirring, maintaining sufficient agitation to break up initial agglomerates. To reduce dust, consider adding kaolin as a prepared slurry (e.g., 50% solids paste).
Furthermore, in terms of sequence, kaolin should be dispersed before adding primary thickeners and electrolytes, as high viscosity or high ionic strength can hinder clay deagglomeration.
Shear Conditions
Kaolin particles may initially exist as weak aggregates, requiring sufficient mechanical shear for breakdown.
A combination of propeller stirring and homogenization is typically used: first use a paddle blade for low-speed stirring to wet and disperse the powder, then switch to medium-high speed stirring (e.g., 300–800 rpm, depending on scale) for over 5 minutes to uniformize the suspension. If possible, subsequent high-shear treatment (e.g., 3000–5000 rpm rotor-stator homogenizer) for 1–3 minutes can significantly reduce fine agglomerates and improve slurry stability.
If system viscosity is too high for effective homogenization, mild heating (e.g., 40°C) or pre-addition of some surfactants can aid wetting and dispersion.
Milling equipment (e.g., ball mill, colloid mill) is generally not used for kaolin dispersion in cosmetics, as excessive shear can potentially reduce particle size and increase viscosity. Overall, thorough wetting and swelling via medium-high speed stirring, supplemented by short-duration high-shear homogenization, is an effective SOP for kaolin dispersion.
3. Compatibility with Formulation Components
Compatibility with Surfactants
Kaolin interacts differently with surfactants of varying charges, requiring consideration during formulation design.
Anionic Surfactants (e.g., SLS, Soap Bases): Generally good compatibility. The negative ions from anionic surfactants in water may adsorb onto the positively charged edges of kaolin, but since kaolin is overall negatively charged at typical alkaline pH and anionic surfactants themselves provide dispersive stability, kaolin usually remains well-suspended in anionic surfactant systems.
Amphoteric/Non-ionic Surfactants (e.g., Cocamidopropylamine Oxide, APG):Usually do not strongly affect kaolin dispersion. Amphoteric surfactants may carry different charges depending on pH, but generally have limited impact on the clay network at low concentrations and can be used as auxiliary wetting agents. Non-ionic surfactants primarily reduce water’s surface tension to aid wetting and do not cause flocculation; they can even help stabilize the suspension.
Cationic Surfactants (e.g., Quaternary Ammonium Conditioning Agents): Require special attention. Cationic surfactants are strongly adsorbed by kaolin (due to the generally negative charge on kaolin surfaces and interlayers). This leads to two potential issues: 1) The effective concentration of the cationic surfactant in the formulation decreases (adsorbed/”eaten” by the clay), potentially impacting its efficacy. 2) Cations can bridge clay particles, causing flocculation and a sharp increase in viscosity. Studies show that in kaolin-cationic surfactant systems, a clay-surfactant composite network forms, increasing yield stress.
Therefore, in formulations requiring both cationic surfactants (e.g., cationic emulsifiers, hair conditioners) and kaolin, the system may thicken and stability may suffer. If coexistence is necessary, it is advised to: (1) Minimize direct contact, e.g., disperse kaolin in the water phase and add the cationic surfactant pre-emulsified with the oil phase at low temperature. (2) Pre-test the impact of different addition sequences on system viscosity. (3) If needed, use sequestrants (e.g., polyphosphates) to pre-treat the kaolin surface, occupying sites to prevent cationic adsorption.
Effect of Electrolytes, Multivalent Ions
Many formulations contain electrolytes, whose ions can weaken the charge repulsion between kaolin particles, causing flocculation. Divalent cations like Ca²⁺, Mg²⁺ are particularly effective, potentially bridging kaolin particles, neutralizing negative charges on silicate faces and anionic surfactants, turning the suspension into a gel. Experience shows that >0.1% concentration of divalent salts can significantly increase the viscosity and yield stress of kaolin pastes.
Monovalent ions like Na⁺ have a relatively smaller impact, but high NaCl concentrations (>1%) can also compress the double layer, inducing mild flocculation. Therefore, when using kaolin in formulations containing salts (e.g., MgCl₂ in Dead Sea mud masks), consider reducing other thickeners to avoid excessive viscosity, or introduce hydrophobic-treated kaolin (less sensitive to ions) as a partial substitute.
pH also affects ionic states: at high pH (>8), most metal ions form hydroxide precipitates or complexes, and negatively charged kaolin can be more stable; around neutral to slightly acidic pH, metal ions exist and promote flocculation. Therefore, when adjusting pH, aiming for neutral to slightly alkaline is recommended for stable kaolin dispersion (unless product specifics require acidity).
Polymer Colloids
Kaolin often coexists with thickening polymers to achieve the desired texture, but they can interact synergistically or competitively, requiring specific analysis.
Guar Gum, Xanthan Gum (Anionic Polysaccharides): These gums can entangle kaolin particles, increasing network strength, manifested as increased viscosity and thixotropy – a synergistic thickening effect. Xanthan gum solutions are inherently thixotropic; adding kaolin superimposes the networks, forming a stronger gel at rest. This synergy is beneficial in masks: a small amount of xanthan gum (0.2–0.5%) can prevent kaolin mud masks from dripping and improve spreadability. However, gum quantity must be controlled, as excess can reduce the tightening effect of the mask upon drying.
Cellulose Derivatives (HEC, CMC, etc.): Non-ionic or anionic cellulose polymers primarily stabilize kaolin suspensions by increasing continuous phase viscosity. There’s no specific incompatibility, but high concentrations of cellulose might encapsulate clay particles, delaying settlement but also weakening the clay’s inherent thixotropy. It’s advised to add cellulose solutions after kaolin is fully dispersed; otherwise, the high-viscosity environment hinders deagglomeration.
Acrylic Rheology Modifiers (e.g., Carbomer, Anionic): In its unneutralized state, Carbomer is a weak gel, and adding kaolin slightly thickens it. However, once neutralized (pH ~7), Carbomer chains expand, releasing anions which can electrostatically interact with the positively charged edges of kaolin, potentially causing some flocculation or phase separation. Therefore, adding kaolin to Carbomer-based creams requires caution; pre-testing is needed to adjust Carbomer levels or consider alternative thickeners (e.g., Acrylates/Steareth-20 Methacrylate Copolymer, more ion-tolerant).
Montmorillonite/Bentonite Clays: Kaolin is sometimes combined with a small amount of bentonite (5–10%) in mud masks, utilizing bentonite’s high swelling capacity to build structure and enhance system viscosity and stability. Kaolin-Bentonite mixed systems form a hierarchical structure: bentonite provides the skeletal viscoelasticity, and kaolin fills and reinforces the framework.
Organic Colloidal Particles (e.g., Emulsion Microspheres, Microcapsules): Kaolin generally has no direct interaction, but note that kaolin might adsorb surfactants or charged molecules from the microcapsule wall material, potentially causing microcapsule aggregation.
Combination with Pigments/UV Filter Powders
Kaolin is often combined with other powders in color cosmetics and sun care. Rational combinations can yield synergistic effects.
With Titanium Dioxide/Zinc Oxide: Physical sunscreens tend to agglomerate and settle at high concentrations. Adding kaolin helps separate these high-refractive-index particles, promoting uniform dispersion and reducing density differences to prevent settling. Kaolin can also adsorb some oil phase, reducing the agglomeration tendency of ZnO, etc., in oils.
With Color Pigments (Iron Oxides, Ultramarines, etc.): Kaolin is inorganic and stable, unlikely to react chemically with color pigments. However, its adsorptivity might capture organic surface treatment agents. For instance, treated pigments (e.g., silane-treated black iron oxide) might lose their treatment agents to the clay upon contact, reducing the pigment’s surface affinity and making dispersion difficult. Therefore, if using significant kaolin, color pigments are best chosen as untreated or pre-coated with resin types to avoid mutual effects on color strength.
With Pearlescent Pigments: Pearlescent pigments (mica/glass coated with TiO₂) are common in highlighters. Introducing kaolin requires caution as its matte nature can diminish the pearlescent shine. Thus, kaolin is typically avoided in highlighter products or used in minimal amounts for tactile adjustment. If a slight control over application is desired, larger particle size kaolin can be used to minimize impact on gloss.
With Spherical Filler Powders (PMMA/Nylon Powder, etc.): Kaolin can be used alongside organic microspheres to enhance powder packing density and oil control. However, kaolin is denser and tends to settle in the bottle, while organic microspheres are lighter and may float. Suspending agents or surface treatment to equalize densities might be necessary.
Optimizing Kaolin Classification for Performance
The effectiveness of kaolin in these diverse applications hinges on its particle size distribution. Implementing precise kaolin classification during processing, potentially utilizing advanced systems like the HTS315 air classifier, ensures consistent performance. Proper kaolin classification directly influences key properties like oil absorption, texture, coverage, and suspension stability, allowing formulators to select the ideal grade for each specific cosmetic application, from lightweight powders to rich mud masks.
III. Quality and Safety Checklist
To ensure the quality and safety of kaolin raw materials and finished products containing kaolin, key inspection points are listed below:
Raw Material Purity Control: Each batch of kaolin should be accompanied by a certificate of analysis confirming the absence of impurities exceeding limits. Particularly, free crystalline silica (quartz) content must be very low (almost absent in washed grades) to avoid respirable silica dust hazards. Heavy metal impurities (Pb, As, Cd, Hg, etc.) must comply with regulations.
Microbiological Risk & Control: Although an inorganic mineral, kaolin can carry desiccant-resistant bacterial spores. Require total viable count <1000 CFU/g, molds and yeasts <100 CFU/g, and absence of specified pathogens upon purchase. Kaolin treated with γ (gamma)irradiation or high-temperature calcination can be selected. In finished formulations, note the potential adsorption of preservatives by kaolin (some preservatives like quaternary ammonium compounds can be adsorbed and inactivated by clay).
Product Efficacy Consistency: As kaolin is a natural mineral, particle size, whiteness, and oil absorption value may vary slightly between batches. Establish internal quality standards, e.g., D50 variation range ±X µm, whiteness ±Y, to ensure consistent product performance across batches.
By following the above checklist, systematic quality control from raw material procurement, production operations, to finished product safety can be ensured, making kaolin-containing cosmetics safe, effective, and compliant with regulatory requirements.
Partner with Epic Powder
Achieving the precise particle size distribution critical for high-performance cosmetic kaolin requires advanced technology. At Epic Powder, our HTS315 Air Classifier is engineered to deliver unmatched precision in kaolin classification. We can efficiently process 400-mesh kaolin into D97 5.80 µm and D50 15.20 µm ultrafine powder. This precise control directly enhances key properties like skin feel, opacity, and oil absorption. It enables you to develop superior cosmetic products.
Unlock the potential of your kaolin-based formulations. Contact us today to learn how our HTS315 air classifier and expertise in kaolin classification can benefit your production process.

